Medical research safety is paramount for protecting patients involved in clinical studies, ensuring their rights and welfare are upheld throughout the research process. The ongoing dialogue about patient safety in clinical research has gained urgency, especially regarding the impact of funding cuts on research. These budget reductions can lead to disruptions in essential oversight mechanisms like Institutional Review Boards (IRBs), which are critical for maintaining research ethics and participant safety. Without adequate funding, the oversight capabilities for clinical trials may diminish, posing risks not only to participants but also to public trust in scientific inquiry. As discussions revolve around NIH funding and ethics, it’s clear that robust financial support is indispensable for safe and responsible medical research practices.
Ensuring the security and well-being of individuals participating in medical trials is a vital aspect of healthcare research. This concept, often referred to as participant protection in human studies, highlights the importance of maintaining rigorous oversight mechanisms, such as IRBs and other ethical review boards. The current environment of reduced financial resources poses threats to the quality of clinical trial oversight, which could lead to unintended consequences for trial volunteers. When funding is slashed, it often jeopardizes the ethical considerations fundamental to maintaining participant safety in research initiatives. Conversations surrounding the fiscal health of medical research increasingly underscore the need for stable support structures to navigate clinical research and safeguard the interests of all involved.
The Crucial Role of Funding in Patient Safety in Clinical Research
Funding plays a pivotal role in maintaining patient safety in clinical research. When research is adequately funded, institutions can ensure that robust protocols are in place for monitoring and protecting participants through ethical standards set by Institutional Review Boards (IRBs). These boards are crucial for navigating the intricacies of research ethics, as they review studies for compliance with rigorous safety standards, informed consent processes, and risk assessments. Inadequate funding puts pressure on these safeguards, making it challenging for researchers to maintain comprehensive oversight, thereby jeopardizing the safety of participants who put their trust in the research process.
Furthermore, the impact of funding cuts extends beyond immediate safety measures; it affects long-term trust between researchers and the community. When funding is disrupted, as seen with the recent federal grant freeze, research studies may face delays, cancellations, or reduced oversight capacity, which can lead to adverse outcomes for participants. These funding challenges can result in a decrease in transparency and scrutiny, creating a ripple effect of uncertainty, which ultimately undermines public confidence in the clinical research process. Addressing these funding issues is essential to uphold the integrity and safety of patient involvement in research.
The Impact of IRB Oversight on Research Ethics
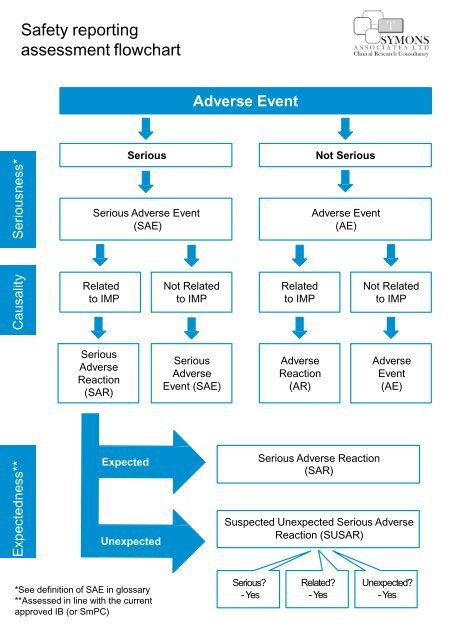
IRBs serve as the backbone of ethical oversight in research, ensuring that studies meet stringent ethical guidelines designed to protect patients. They review protocols that outline the objectives, methods, and potential impacts of research, assessing whether they respect the rights and welfare of all participants. The process includes evaluating informed consent procedures, participant safety protocols, and plans for handling adverse events. This level of oversight is essential, especially in complex studies involving vulnerable populations, where the risks may be amplified. Without the support and guidance of IRBs, researchers could unintentionally compromise participant safety or overlook critical ethical considerations.
Moreover, IRB oversight helps foster an environment of accountability and education among researchers. By providing training and resources on ethical research practices, these boards equip researchers to navigate the complexities of their studies while ensuring compliance with legal standards and ethical norms. This proactive approach minimizes the risk of ethical breaches, which not only protects participants but also enhances the overall credibility of the research enterprise. As funding challenges threaten the sustainability of these essential organizations, ensuring robust IRB support becomes even more crucial for maintaining high ethical standards in clinical trials.
Challenges Arising from Funding Cuts in Clinical Research
The recent cuts to research funding pose significant challenges for academic institutions and clinical research facilities, particularly in how they manage ongoing studies. As funding dries up, it becomes increasingly difficult for institutions to sustain the necessary infrastructure for conducting ethically sound research. This includes maintaining the essential functions of IRBs, which rely on adequate resources to perform comprehensive reviews and facilitate oversight. The resulting delays and the inability to recruit new sites for ongoing studies can lead to interrupted participant care and hinder advancements in patient treatment options.
Furthermore, the cancellation or halting of research projects can have broader implications for scientific progress. When collaboration across multiple research sites becomes stymied due to funding shortages, it can lead to fragmented data and lack of cohesion in the research community. This reduces synergy in knowledge sharing among researchers and severely impacts the quality of research outcomes. Ultimately, the ripple effects of disrupted funding make it harder to achieve meaningful breakthroughs that can enhance public health and safety.
NIH Funding and Its Role in Supporting Patient Safety
NIH funding plays an instrumental role in supporting patient safety within clinical research by providing the financial backing necessary for standards of care, oversight, and participant protections. NIH-funded grants often come with strict guidelines stipulating that research must pass through an IRB, ensuring that ethical dimensions of the studies are respected. These funds facilitate comprehensive training for research teams about the importance of ethical standards in protecting patient rights and welfare, which in turn leads to more reliable and ethically conducted research.
Moreover, NIH’s commitment to supporting multi-site collaborations through initiatives like the single IRB (sIRB) mandate signifies an effort to simplify ethical oversight while maintaining rigorous standards of safety and compliance. By streamlining processes for collaborative research studies, NIH enhances the ability of institutions to work together effectively, ensuring that patient safety remains a priority across all participating sites. Without sustained NIH funding, however, the achievements in patient protection and ethical research processes could face setbacks, which would ultimately compromise the safety of research participants.
The Importance of Ethical Guidelines in Clinical Trail Oversight
Ethical guidelines are paramount in ensuring that clinical trial oversight prioritizes patient safety above all else. These guidelines, established by organizations such as the FDA and supported by institutional review boards, dictate the nuances of conducting research aimed at human subjects. They include critical elements such as informed consent, risk assessment, and protocols for addressing adverse events. By adhering to these guidelines, researchers can minimize the potential for harm and ensure that participants are safeguarded throughout the research process.
The oversight provided by these ethical guidelines fosters a culture of respect and responsibility within the research community. It emphasizes the moral obligation researchers have to protect participants, particularly vulnerable populations who may be at a greater risk. As funding cuts place strain on research institutions, it becomes even more vital to uphold these ethical standards to maintain the integrity of clinical trials. Failure to do so could not only result in detrimental outcomes for research participants but could also diminish public trust in the entire research ecosystem.
Healthcare Innovation and Its Dependency on Robust Research Funding
Healthcare innovation is intrinsically tied to the availability of robust research funding, which enables the exploration of new treatments, therapies, and technologies. Funding supports the scientific inquiry necessary to advance knowledge, which often translates into improved patient outcomes. A well-funded research environment nurtures creativity and collaboration, allowing for novel ideas to flourish and be tested. Conversely, reductions in funding stifle innovation, leading to fewer breakthroughs in medical care and potentially delayed improvements in patient safety.
In the absence of substantial funding, researchers may be forced to prioritize lower-risk studies that yield less impactful results, inhibiting the progression of groundbreaking research. A lack of financial support can also discourage talented researchers from entering the field, thereby limiting the pool of innovative minds dedicated to solving pressing health challenges. Thus, to secure a future of healthcare advancements and maintain or enhance patient safety, sustained investment in medical research funding is essential.
Addressing Ethical Complications in Medical Research
Ethical complications in medical research have historical relevance, underscoring the importance of stringent oversight and ethical standards. Past transgressions in clinical research, such as the Tuskegee Study and the Willowbrook hepatitis experiments, spotlight the necessity for IRB oversight that prioritizes the rights and welfare of participants. Current ethical frameworks emerged as safeguards to prevent these violations from recurring, emphasizing informed consent, risk-benefit analysis, and equitable participant selection.
As research evolves, so too do the ethical dilemmas that researchers encounter, particularly with emerging technologies and methodologies. Researchers must navigate issues like data privacy, genetic research challenges, and the moral implications of potential artificial intelligence applications in clinical settings. Addressing these complications requires ongoing dialogue within the research community and continuous adaptation of ethical guidelines. Institutions must remain vigilant in their oversight efforts to support ethically sound research, ensuring that participant safety is paramount.
Building Trust in Clinical Research through Transparency
Trust is a cornerstone of clinical research, yet recent funding cuts threaten to erode this vital foundation. Transparency in the research process is essential for participants, healthcare providers, and the public to maintain confidence in the integrity of studies. When funding disruptions occur, leading to halts in ongoing research, it can cause participants to question the commitment to safeguarding their rights and well-being. By fostering open lines of communication and demonstrating accountability, researchers can rebuild trust and enhance participation in future studies.
Establishing a culture of transparency also involves clear reporting of research findings and ethical practices. Engaging the community in the research process through public forums and discussions can facilitate understanding and support for clinical trials. As researchers showcase the safety protocols enforced by IRBs and the ethical considerations woven into study designs, it strengthens public confidence in the medical research framework. Trust fosters participant engagement and is essential for advancing research that can lead to meaningful health improvements.
Community Engagement as a Pillar for Ethical Clinical Trials
Community engagement plays a crucial role in fostering ethical clinical trials. Involving community members in the research process ensures that studies are designed with the needs and concerns of participants in mind, thereby increasing trust and participation. Engaging community representatives in discussions about research goals, methods, and potential impacts leads to research that is more in tune with the communities being studied, ultimately enhancing ethical standards and participant safety.
Moreover, community engagement helps to combat historical mistrust stemming from past abuses in research. By establishing a collaborative partnership with community members, researchers can work to ensure that studies are inclusive and equitable, particularly for marginalized populations. This approach not only supports the ethical integrity of the research process but also strengthens the community’s confidence in participating, paving the way for future innovations in medical research that prioritize patient safety and welfare.
Frequently Asked Questions
What is medical research safety and why is it important?
Medical research safety refers to the practices and protocols implemented to protect the rights and welfare of participants involved in clinical trials. It is crucial to ensure ethical treatment, informed consent, and to minimize risks associated with research participation. Safeguarding medical research safety is vital for maintaining public trust, enhancing participant recruitment, and ensuring the integrity of research findings.
How does patient safety in clinical research impact research outcomes?
Patient safety in clinical research is fundamental as it influences the quality and reliability of research outcomes. When safety protocols are prioritized, participants are more likely to have positive experiences, which facilitates recruitment and retention. Prioritizing patient safety also fosters a trustworthy environment that can lead to better collaboration between researchers and communities, ultimately enhancing the credibility of clinical findings.
What are the consequences of funding cuts on patient safety in medical research?
Funding cuts can severely disrupt patient safety initiatives in medical research by limiting resources available for oversight, training, and facilities. Such reductions may lead to fewer IRB reviews, less comprehensive safety monitoring, and ultimately increase risks for participants. Research institutions may struggle to maintain ethical standards, which can directly impact the safety and welfare of research volunteers.
What role do IRBs play in ensuring medical research safety?
Institutional Review Boards (IRBs) are charged with reviewing research proposals to ensure the safety of participants in clinical trials. They assess ethical considerations, the informed consent process, and monitor ongoing studies to mitigate risks. By providing this oversight, IRBs play a crucial role in safeguarding patient safety, thereby enhancing the integrity of medical research.
How does NIH funding contribute to patient safety in medical research?
NIH funding is essential for supporting comprehensive research oversight, including the implementation of safety protocols necessary for protecting participants. It ensures that studies submit to IRB reviews and adhere to federal regulations, which are critical for maintaining ethical standards and promoting patient safety during clinical trials. The support from the NIH also enables extensive collaboration among institutions, enhancing the overall safety framework.
What are the ethical implications of poor oversight in clinical trials?
Poor oversight in clinical trials can lead to significant ethical implications, including violations of informed consent and increased risk of harm to participants. Historical events have shown that inadequate supervision can result in exploitation, loss of trust in research, and long-lasting damage to public perception of medical research safety. Upholding rigorous oversight is essential for maintaining ethical standards and ensuring participant safety.
How can researchers ensure compliance with safety protocols in medical research?
Researchers can ensure compliance with safety protocols by adhering to established guidelines set by IRBs, conducting thorough risk assessments, providing comprehensive training for staff, and maintaining open lines of communication with participants. Regular audits and monitoring of the research process also play a crucial role in ensuring that safety measures are effectively implemented and followed throughout the duration of the study.
What measures can be taken to improve clinical trial oversight?
To improve clinical trial oversight, measures such as increasing funding for IRB operations, facilitating ongoing education for research staff, and implementing robust systems for tracking adverse events can be adopted. Enhanced collaboration among institutions and transparent reporting mechanisms are also critical steps towards strengthening oversight and ensuring the safety of patients participating in medical research.
| Key Points | Details |
|---|---|
| Impact of Funding Cuts | Federal funding cuts exceeding $2 billion have disrupted safety efforts for patients in medical research. |
| Role of SMART IRB | SMART IRB streamlines oversight of multi-site studies, protecting research participants’ rights and safety. |
| Institutional Review Boards (IRBs) | IRBs ensure compliance and protect human participants through review, oversight, and education. |
| Historical Context | Historical abuses have led to the development of stringent oversight systems to ensure ethical conduct in research. |
| Effects on Research | Funding cuts jeopardize ongoing studies, risking participant safety and eroding public trust in medical research. |
Summary
Medical research safety is critically endangered by recent funding cuts, which have stymied essential oversight mechanisms that protect patient participants in clinical studies. The comprehensive review and ethical oversight provided by Institutional Review Boards (IRBs) are crucial in ensuring participant safety. Decreased funding not only halts ongoing research projects but also undermines public confidence in the medical research community, potentially deterring future innovations. To ensure the health and welfare of participants, it is imperative to restore adequate funding and support for medical research initiatives.